2px
Option (i) is the answer.

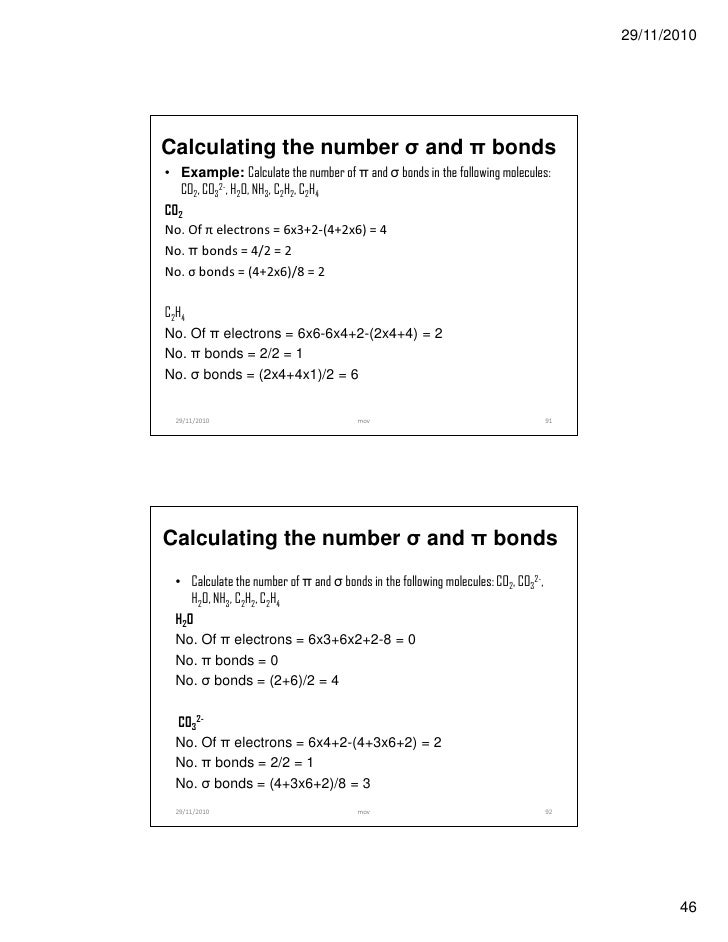
2px. If Z is = or > 8. Molecular orbital (MO) theory:. These electrons are the cause of O 2 being a triplet diradical in the ground state (indicated as 3 O 2).
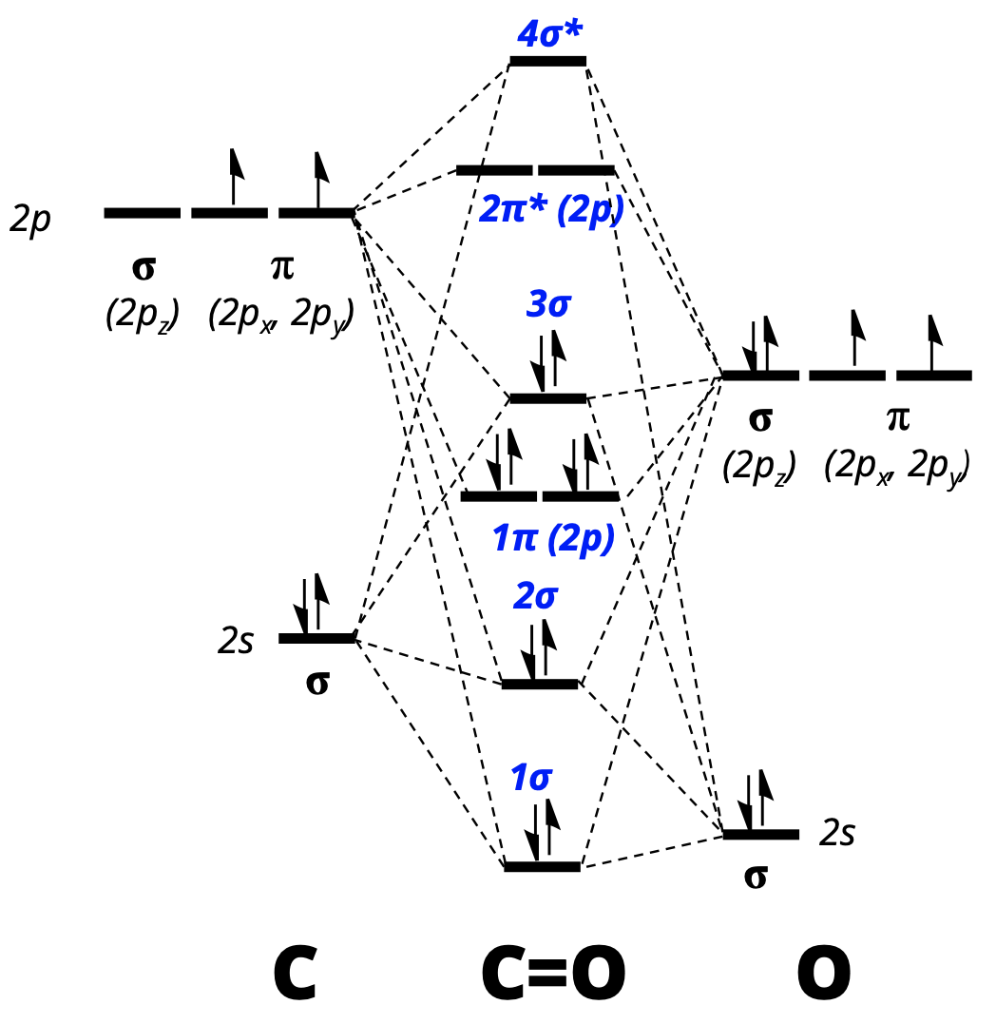
(ii) He 2 is not stable but He 2+ is expected to exist. 2 electrons in each bond + lone pairs in remaining sp2 hybrids on O's = 18 electrons. The molecular orbital configuration = KK σ(2s) 2σ * (2s) 2 π (2px) 2 π (2py) 2 σ (2pz)2 Thus, the MO diagram is :.
5.111 Lecture Summary #14 October 13, 09 14.5 2p z 2p y 2p x 2p z 2p y 2p x π 2px. Mixture of both molecular orbitals results in an electron transfer from the d xz and d yz orbitals of Rh atom to π 2px * and π 2py * orbital. (1) 0.10 m KOH (2) 0.15 m CH 3 OH (3) 0.05 m BaCl 2 (4) 0.05 m NH 4 NO 3 (5) 0.10 m K 2 SO 4.
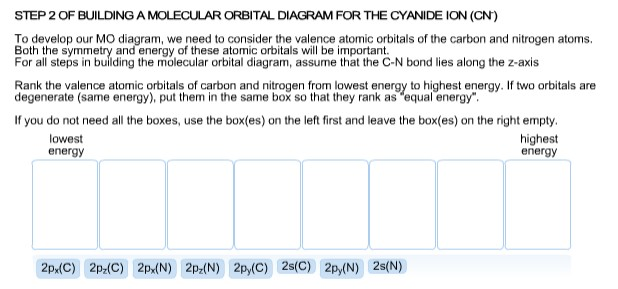
Orbitals having nearly same energy are called degenerate orbitals. Y z x y x C N σ 2pz. Is the bond distance of neutral CN smaller or larger than 1.15 Å?.
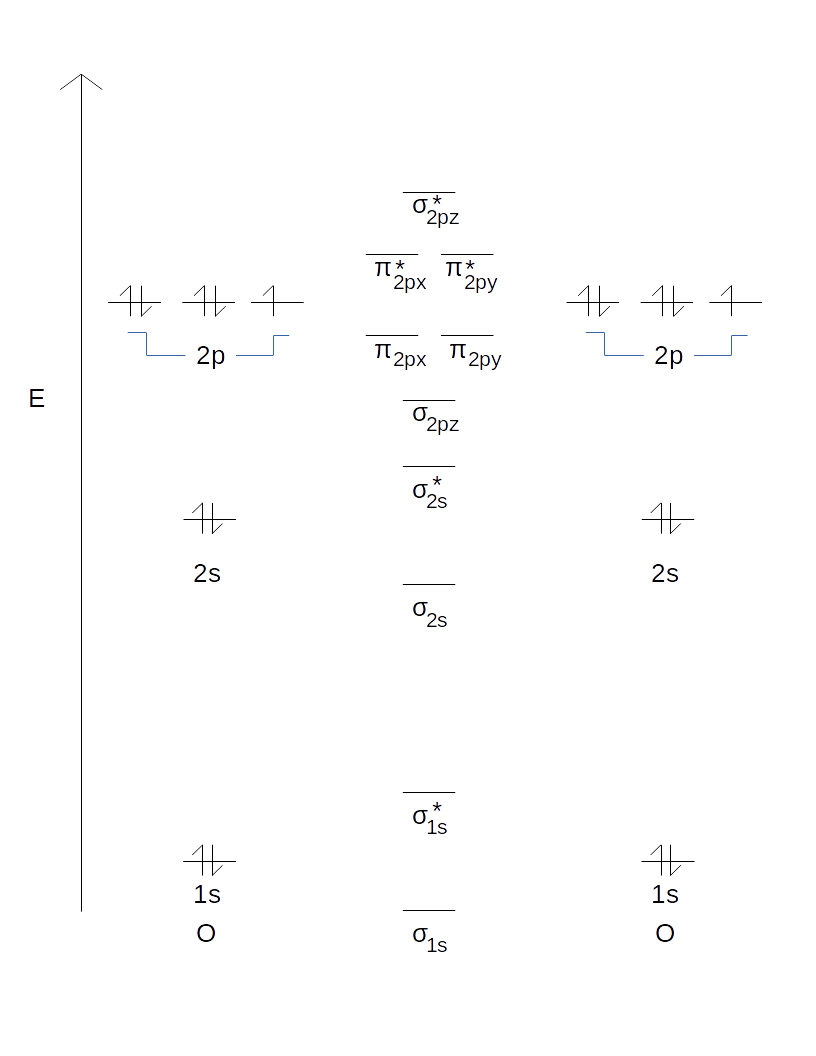
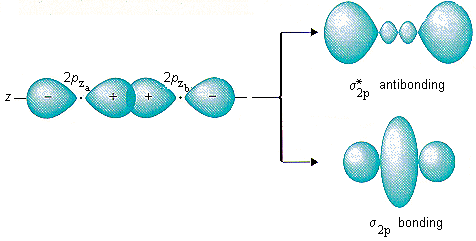
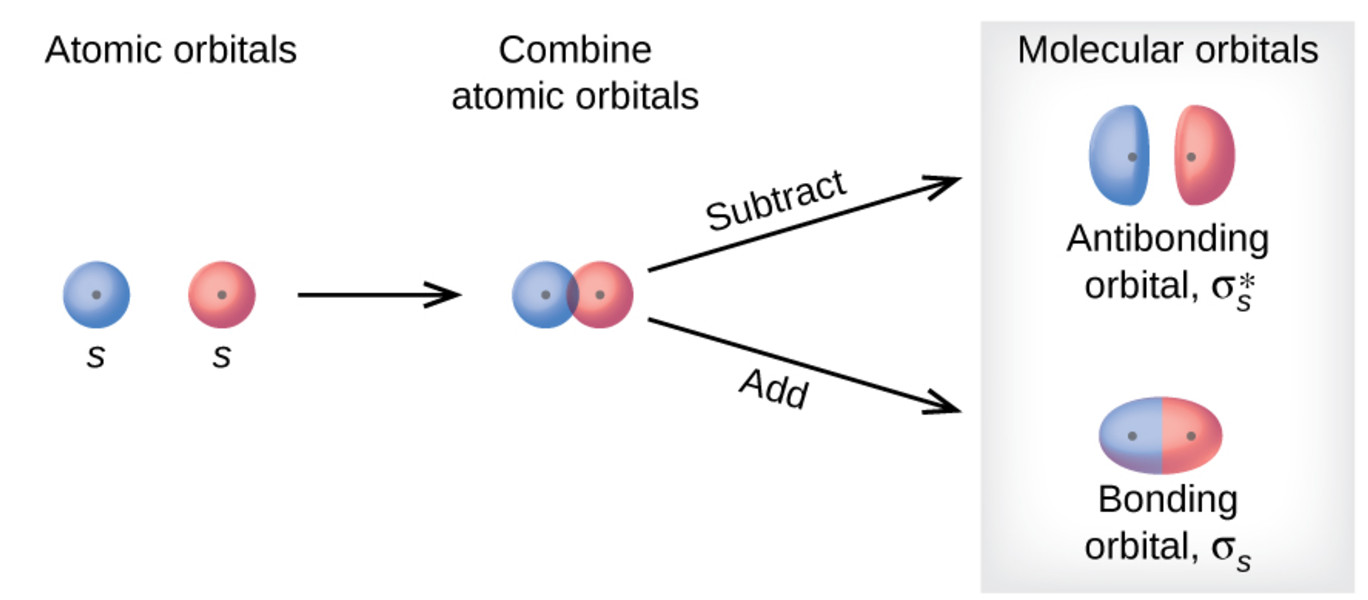
The atomic orbitals combine (overlap) to form a new orbital known as molecular orbital. In case of a photon, if it is assumed to have wave character, its energy is given by. Write the π2px and π2px* molecular orbitals in terms of the 2px atomic orbitals.
Asked on May 01, by Anudeep Gar. Click here👆to get an answer to your question ️ Number of nodal planes in π∗2px are. For Li-N:σ1s < σ∗1s < σ2s < σ∗2s < π2px, π2py < σ2pz < π∗2px, π∗2py < σ∗2pz for O-Ne:σ1s < σ∗1s < σ2s < σ∗2s < σ2pz < π2px, π2py < π∗2px, π∗2py < σ∗2pz Electronegativity Info atom Electronegativity B 2.04 C 2.55 N 3.04 O 3.44 F 3.98 Cl 3.16 PERIODIC TABLE H 1.0079 1 He 4. 2 Be 9. 4 Li 6.941 3 B.
σ1s < σ*1s < σ2s < σ*2s <σ2pZ<(π 2pX= π 2py) < (π*2px= π*2py)<σ*2pZ However, this sequence of energy levels of molecular orbitals is not correct for the remaining molecules Li2, B2,C2 , N2. Both π(2px) and π⋆(2px)MO’s have one nodal plane each. The new molecular orbitals are formed as follows:.
The highest occupied molecular orbital (HOMO) of O 2 is a pair of degenerate antibonding π orbitals, π 2px * and π 2py *, which are both singly occupied by spin unpaired electrons. Which of the following processes is not exothermic?. Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule.
The electron configuration of "N"_2 is (σ1s)^2(σ^"∗"1s)^2(σ2s)^2(σ^"∗"2s)^2(π2p_x)^2(π2p_y)^2(σ2p_z)^2 > The molecular orbitals of "N"_2 are formed by overlapping the atomic orbitals of the "N" atoms. Since the core orbitals are full occupied , we will consider for the valence shell (2nd shell). Y z x y x C N π* 2px.
Draw diagrams similar to those in the model to show how the π 2px and π 2px * molecular orbitals. So, according to Hund's rule the last two electrons are filled singly. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals.
Determine the bond order in a molecule or ion with:a. The answer is c because it is the most stable moleculebecause of addition of an electron in b. Both π(2px) and π*(2px) MO's have one nodal plane each.
During the change of O₂ to O₂⁻, -. The relative energies of the σ 2pz orbital compared to the π 2p orbitals depends on the Z value of the atoms. (σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(σ2p)2(π2p)4(π*2p)2 n = (σ1s)2(σ2s)2(π2p)4(σ2p)2= 10 n* = (σ.
The reason that it is paramag. (i) Be 2 is not a stable molecule. σ1s σ*1s 1s1s 2s2s σ2s σ*2s σ2pz σ*2pz 2p2p π2px π2py π*2px π*2py 2pz 2py2px 2px2py2pz Orbital atom O Orbital molekul O2 Orbital atom O Konfigurasi elektron Atom 8O = 1s2 2s2 2p4 Diagram Korelasi Molekul O2 O2 yang konfigurasi elekron:.
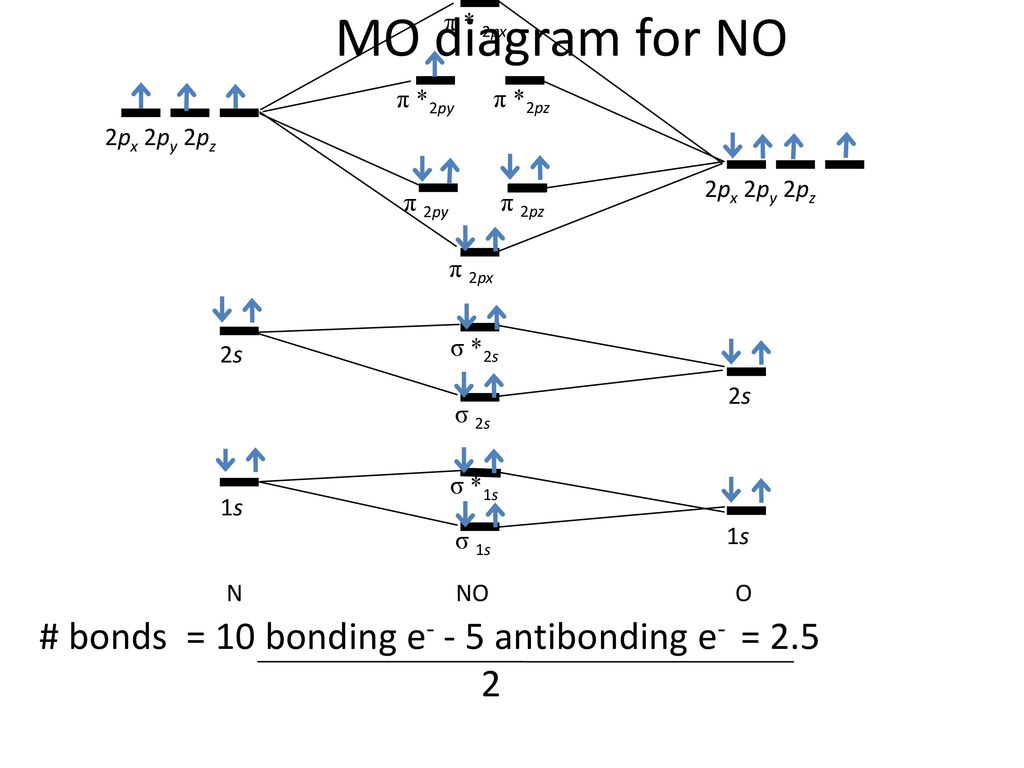
The (NO) molecule has one unpaired electron from ( π * 2px) corresponding shells. According to MO theory;. The 'p' tells us that the element is found in the p-block which are all of the Groups to the right of the transition.
B 2 2– , e. Which of the following electron distributions among the molecular orbitals best describes the NO molecule?. = ∆E d = 941 kJ/mol O 2 is a _____!.
E) Which (CN-or CN) is paramagnetic and what is its spin-only magnetic moment?. In calculating bond order, we can ignore KK, as it includes two bonding and two antibonding electrons. Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals.
The '3' informs us that the element is in the 3rd Energy Level or row of the periodic table. (a) If both A & R are correct and R is the correct explanation of the assertion. On the other hand, the calculated NBO partial charge on the Rh and W atoms in CO*@O v,b and CO*+O 2 * b may also provide the nature of charge transfer feature about the activation of the O 2 molecule.
Learn vocabulary, terms, and more with flashcards, games, and other study tools. Option (i) is the answer. The HOMO is one of the degenerate π2px,π2py set (a π-bonding MO formed from a combination of 2pxor 2pyorbitals on C) Since this is a homonuclear molecule, there should be equal contributions from the atomic orbitals to the MO.
Molecular N 2 is less reactive than molecular O 2. The result is a slight change in the relative energies of the molecular orbitals where the π 2px, and π 2py orbitals lie at a lower enegy levels than the σ 2pz orbital. (i) NF 3 and BF 3 (ii) BF 4 – and NH 4 + (iii) BCl 3 and BrCl 3 (iv) NH 3 and NO 3 –;.
Isostructural species are those which have the same shape and hybridisation. That electron will constantly fluctuate between both oxygen and decrease the stability. σ(1s) <σ∗(1s) < σ(2s) <σ∗(2s) < π(2px) = π(2py) < σ(2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding ….
When the cations Na+, K+, Rb+, Cs+ are combined with chloride ion in the gas phase to form ion pairs, which pair formation releases the greatest amount of energy?. Here, (σ 1s) 2 (σ 2s) 2 part of the configuration is abbreviated as KK, which denotes the K shells of the two atoms. All MO’s formed by side way overlapping of 2p-orbital.
Carbonate ion, CO3 2-.Regular trigonal planar, equal bonds, all angles 10. (i) Be2 is not a stable molecule. (π2Px)2=(π2Py)2 (π* 2Px)1=(π*2Py)1 4) Bond order = 2 5) Paramagnetic 2.
(ii) He2 is not stable but He2+ is expected to exist. Question from very important topics are covered by NCERT Exemplar Class 11.You also get idea about the type of questions and method to answer in your Class 11th examination. (iv) (π2py ) > (σ2pz) < (π*2px) ≈ (π*2py ) Solution:.
B 2, C 2, and N 2 are best described by this model that includes hybridization. Please explain or I will not rate. Gaseous oxygen is paramagnetic also but is moving too fast to be affected by the magnets.
Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow:. As there is one unpaired electron in π* (2px) so it will be paramagnetic (c)B2 = total no.of electrons = 5 + 5 = 10 = sigma (1s) ^2 sigma* (1s)^2 sigma (2s)^2 sigma* (2s)^2 π (2px)^1. Which of the following statement is not correct from the viewpoint of molecular orbital theory?.
π 2px and π 2py. Ed.) Read for Lecture #14:. O 2+ = = (σ1s) 2 (σ*1s) 2 (σ2s) 2 (σ*2s) 2 (σ2z) 2 (π2px 2 = π2py 2) (π*2px 1) Bond order = 2.5 It is paramagnetic as it contains one unpaired electron.
Which of the following aqueous solutions will have the lowest freezing point?. The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^2 is the element Silicon. (b) If both A & R are correct but R is not the correct explanation of the assertion.
The molecular orbital energy level diagram of N 2 is given in. Since two electrons in oxygen (π* 2px) & (π* 2py) are unpaired, it is paramagnetic in nature. The molecular orbitals are the energy states of a molecule, in.
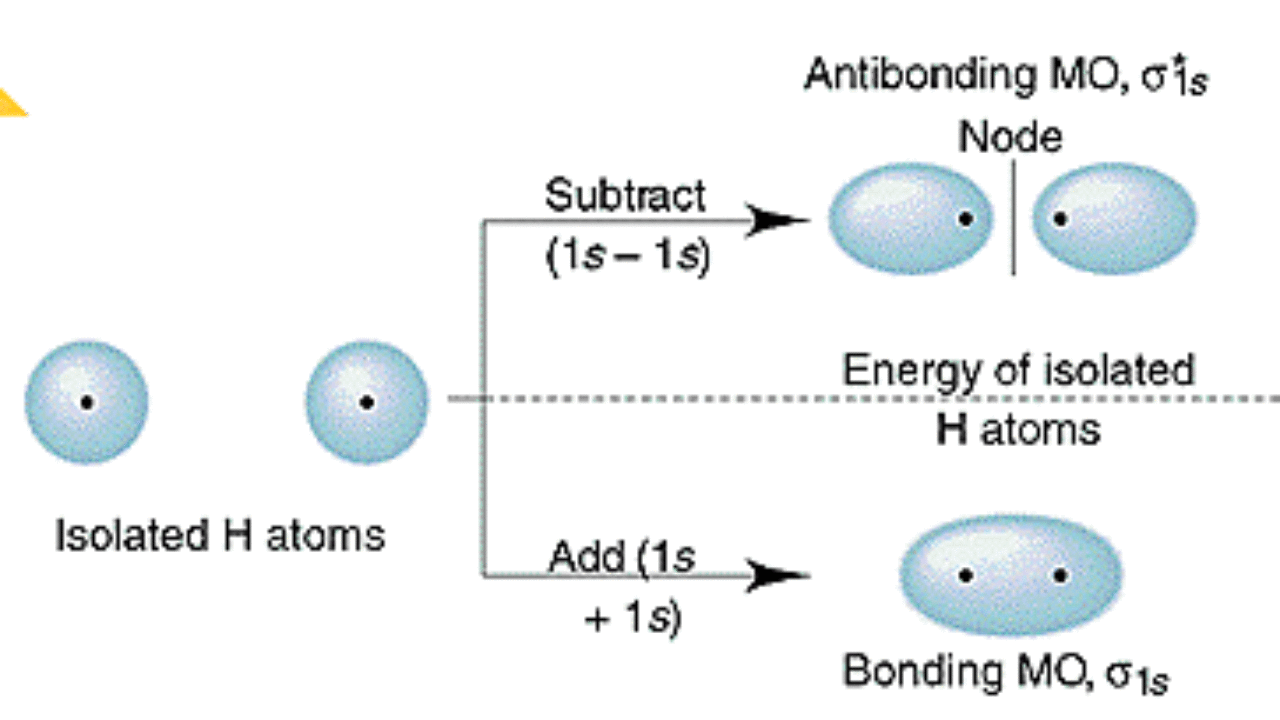
The 1s orbitals form a bonding σ1s and an antibonding σ"*"1s MO. (iv) (π2py ) > (σ2pz) < (π*2px) ≈ (π*2py ) Solution:. As a result, the atomic oritals loose their identity.
Molecular orbital correlation diagram of (OF ‑) ions. Start studying Chemistry 1411- Test 2. S-p separation is small 37.
The 2s orbitals form a bonding σ2s and an antibonding σ"*"2s MO. The bond order shows the number of chemical bonds present between a pair of atoms. Both are highly electronegative atoms which will repel each other and low bond strengthin c.
Which of the following statement is not correct from the viewpoint of molecular. Number of nodal planes in. It is paramagnetic as it contains two unpaired electrons.
KK (σ 1s) 2 (σ 2s) 2 (π 2px) 2 (π 2py) 2 (π 2pz) 2. D) The CN− anion has a C-N bond distance of 1.15 Å. Among the given species identify the isostructural pairs.
2pz and the π* 2px molecular orbitals of CN-on the following coordinate systems. Here, the bond order = 1 / 2 (8-2 ) = 3, the molecule will exist, but there is no unpaired electron in the molecule so it will be diamagnetic. Here we see that, in the antibonding shell, having highest energy, the last two electrons fill singly ,because both the π*(2Px) 1 π*(2Py) 1 orbitals have nearly equal energy.
2s, π 2px = π 2py, σ 2p, π* 2px= π* 2py, σ* 2p (1) 2, 2 (2) 3, 0 (3) 3, 1 (4) 1.5, 2 (5) 2.5, 1 26. Sections 3.4, 3.5, 3.6 and 3.7 – Valence Bond Theory (Same in 5. There is one unpaired electron in HOMO orbital.
Among the given, π 2px and π 2py orbitals are of eqouivalent energy, thus, are called degenerate orbitals. A) KCl B) All release the same amount of energy C) RbCl D) NaCl E) CsCl 2. In case of N 2 molecule :.
Here we have provided NCERT Exemplar Problems Solutions along with NCERT Exemplar Problems Class 11. These hot electrons are reallocated to adsorbed O 2 molecules, generating triplet diradical 3 O 2 formed in degenerate antibonding π 2px and π 2py orbitals, that subsequently is involved in the. The increasing order of energies of various molecular orbitals for O2 F2 is given below :.
The configuration (σ 1s) 2 (σ 1s *) 2 (σ 1s) 2 (σ 2s *) 2 (π 2py) 1 (π 2px) 1 is the molecular orbital description for the ground state ofa. So this molecule obeyed for paramagnetic nature. NCERT Exemplar Class 11 Chemistry is very important resource for students preparing for XI Board Examination.
Derive / Defne de Broglie's equation. The key to deciphering this is to look at the last bit of information of the electron configuration 3p^2. The nitrogen monoxide ( N O) molecule bond order should be calculate and identified.
2(π 2px) 2(π 2py) 2 (σ 1s) 2(σ 1s *) 2(σ 2s) 2(σ 2s *) 2(π 2px) 2(π 2py) 2(σ 2pz) 2 (π 2px *) 1(π 2py *) 1 B.O. Asked Jan 4, 19 in Chemical Bonding and Molecular Structure by Sahida ( 79.5k points) chemical bonding. Use sp2 hybrids on both C and O's.
Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in. (b) π 2px and π 2py (c) π 2px and σ 2pz (d) σ 2px and σ * 2pz. 5.111 Lecture Summary #13 Monday, October 6, 14 Readings for today:.
Section 3.8 – 3.11 Molecular Orbital Theory (Same in 5 th. C and N are joined by a triple bond one s and 2 p's due to high combining capacity of C and small size of N. The bond length of N 2 is shorter than in O 2.
= ∆E d = 494 kJ/mol B.O. 🤓 Based on our data, we think this question is relevant for Professor Cabirac's class at ASU. 1) N = 1s2 2s2 2p3 2) N2 = 14 electrons.
Explain the molecular orbital theory for Nitrogen (N2) molecule.
Http Butane Chem Uiuc Edu Pshapley Genchem2 A6 Book Pdf

Y 2px P N 1st Order Higher Degree Diff Eqn Youtube
2
2px のギャラリー
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures Lecture 14 mo theory Pdf

Molecular Orbital Electronic Configuration For X Anion Is Kk S2s S 2s

Singlet N P P P P Electronically Excited And Singlet Ground State Download Scientific Diagram

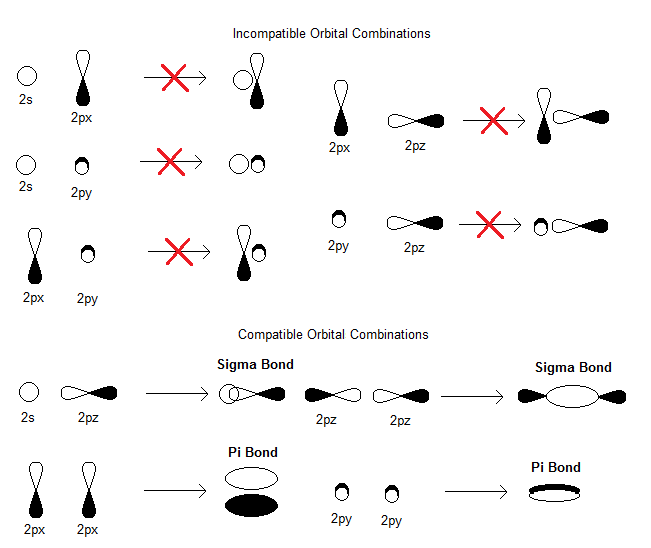
Solved Decide If The Following Pairs Of Orbitals Overlap Chegg Com

How Many Nodal Planes In Antibonding Sigma P Orbital Quora

Penta Aln2 Monolayer A Ferromagnetic Insulator Sciencedirect
Mpl02chyjskjkm

Number Of Nodal Planes In P 2px

Urgentest Find The Number Of Nodal Planes In Pie 2px Are Its Pie Star Two P X Chemistry Chemical Bonding And Molecular Structure Meritnation Com
Q Tbn 3aand9gcss8waok3ahmxp5 Evk18etpm5tqyixradp5tvi0xljxvbchc Usqp Cau

Molecular Orbital Theory Molecular Orbital Theory Describes Covalent Bonds In Terms Of Molecular Orbitals Which Result From Interaction Of The Ppt Download
Http Www Chem Ualberta Ca Htaube 1g 1lecfa10 14po Cbvii Mo Pdf

Number Of Nodal Planes In P 2px Are
People Wou Edu Postonp Ch221 Pdf Summary Mo Theory W16 Pdf

Molecular Orbital Theory Flashcards Quizlet

Number Of Nodal Planes In P 2px Are

Molecular Orbital Theory Mot I2tutorials

B 2 3 3 6 U03c3 2s 2 U03c3 2s 2 U03c0 2px 1 U03c0 2py 1 U03c0 2px Or U03c02py Bonding C 2 4 4 8 U03c3 2s 2 Course Hero
Advanced Organic And Mechanisms Ppt Download

What Is The Order Of Energies Of Various Molecular Orbitals Brainly In

Number Of Nodal Planes In P 2px
How Do You Draw A Molecular Orbital Diagram For A Diatomic Molecule Socratic
Why Do Pi Bonds Restrict Rotation Socratic

The A Dependence Of The A P 2p X Cross Section For Different Assumed Download Scientific Diagram

Ideal And Real Structures Of Different Forms Of Carbon With Some Remarks On Their Geological Significance Journal Of The Geological Society

Null D Lle On An Emple 7 If P Q R Are In A P Then 2px 1 29x 1 27x 1 X 0 Are In A A P B G P For All X 0 C G P
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures Lecture 14 mo theory Pdf
Molecular Orbital A Molecule In Which All The Electrons Are Paired Is Called Diamagnetic
Chemical Bonding Part 1

Number Of Nodal Planes In P 2px Are
Assertion Both P2px And P 2px Molecular Orbital Have One Nodal P
Q Tbn 3aand9gcq7ufxowzic9irhezeespst Rcp7c60lmhbb1xabhznwkqh8rnk Usqp Cau

The Electronic Configurations Of Atomic N And Molecular Nitrogen N 2 Download Scientific Diagram

10 Partial Dos For O2 Adsorbed On Ag156 Calculated With Gs Electron Download Scientific Diagram

Pdf Chemistry I Fall 12 Molecular Orbital Theory Schrenk 11 22 13 Experimental Observation Oxygen Is Paramagnetic How To Explain Patel Harsh Academia Edu
Solved 1a 1b Which Are Pi Symmetric And Which Are Sigm Chegg Com
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures Lecture 14 mo theory Pdf

Figure 2 From Crystal Structure Of Graphite Graphene And Silicon Semantic Scholar

Molecular Orbital Theory Molecular Orbital Theory Describes Covalent Bonds In Terms Of Molecular Orbitals Which Result From Interaction Of The Ppt Download
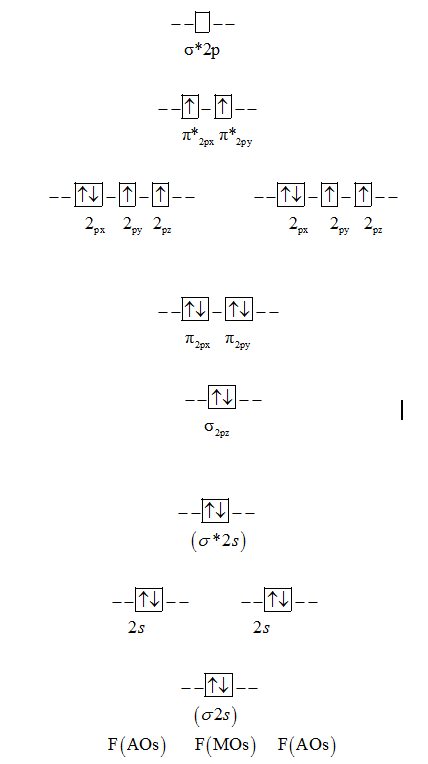
Answered Using The Appropriate Mo Diagram Bartleby

Solved Configuration For Oxygen Molecule Using The Molecu Chegg Com
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures Lecture 14 mo theory Pdf

If The Roots Of The Equation Qx 2 2px 2q 0 Are Real And Unequal Prove That The Roots Of The Equation Brainly In

Chem Brains Atomic Structure Ii Five Marks
Types Of Molecular Orbital Formed Chemical Bonding And Molecular Structure Chemistry Class 11
Molecular Orbital Energy Of Antibonding M O Is Higher Than The Energy Of Atomic Orbitals
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures Lecture 13 mo theory diatomics 17 Pdf
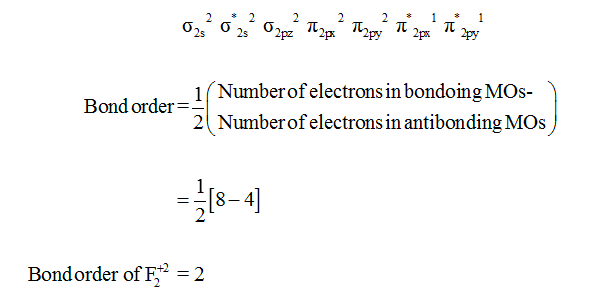
Answered Using The Appropriate Mo Diagram Bartleby

Selected Ks Orbitals As A Function Of The Dimensionless Coordinate Z Download Scientific Diagram
Http Www Chem Ualberta Ca Htaube 1g 1lecfa10 14po Cbvii Mo Pdf

Which Of These Orbitals Has Two Nodes 1 Sigma 1s 2 Sigma 2pz 3 P2px 4 P 2px Brainly In
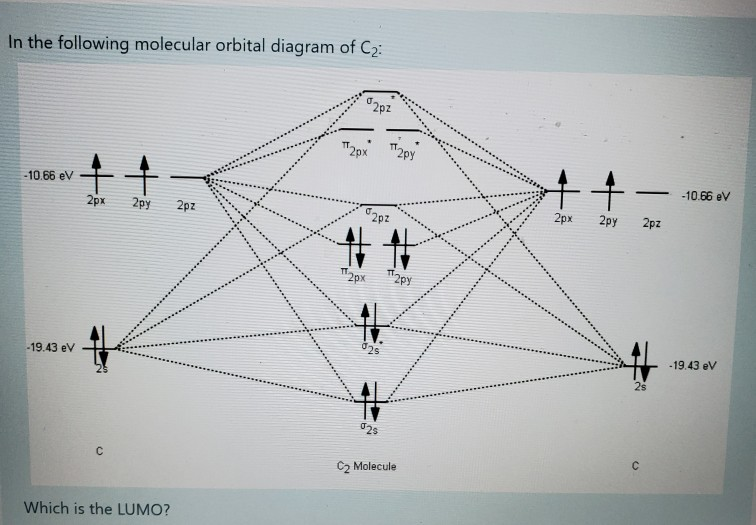
Solved Fill The Mo Diagram Of The Molecule Of Oh H P P 2p Chegg Com
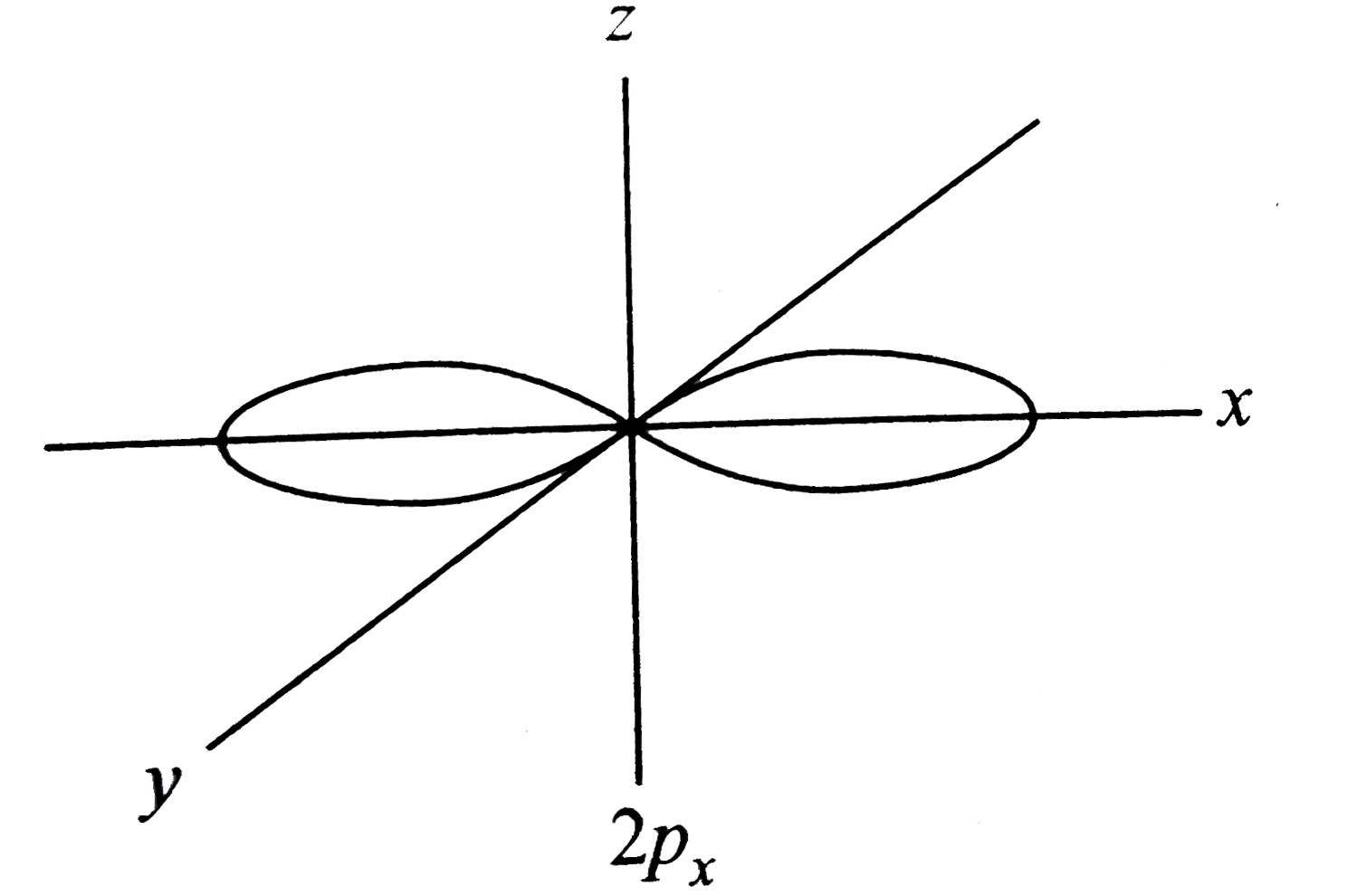
Number Of Nodal Planes In P 2px
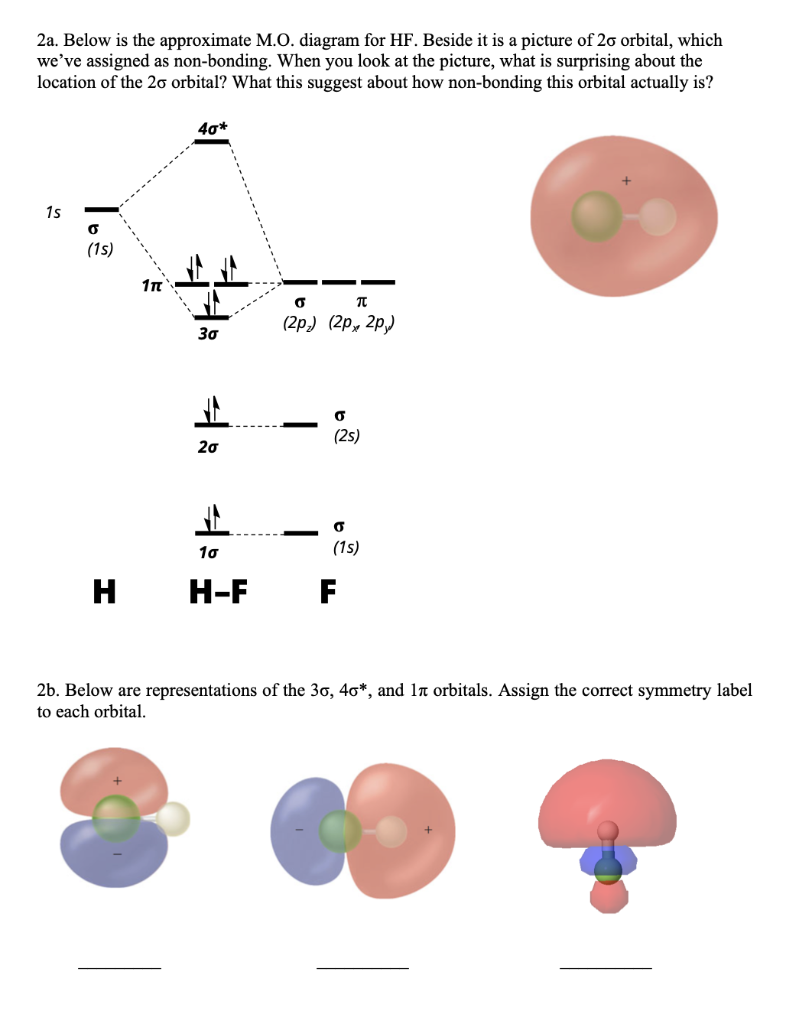
Vhh2fttzk408em

Number Of Nodal Planes In P 2px Are

What Are The Magnetic Quantum Numbers For The Three Real P Orbitals Chemistry Stack Exchange
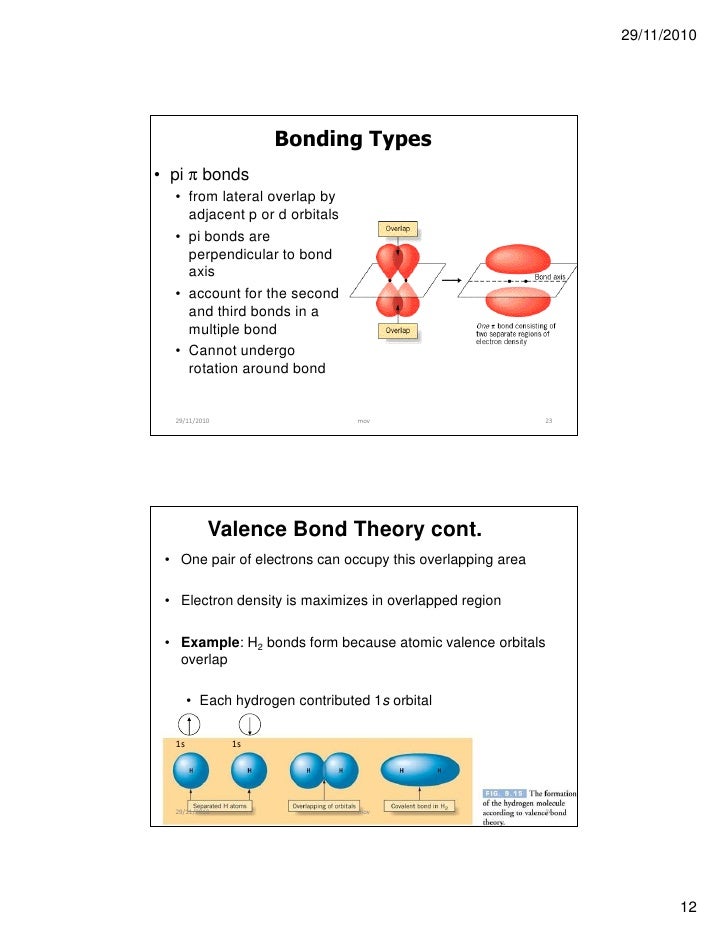
Chemical Bonding Part 1
Q Tbn 3aand9gcry Vts7dc6tpt4v Tcied Ve B9ondwookhju3qrfflwohmvrc Usqp Cau

4x 2 2px 9 0 Find The Vale Of

Nitrogen And Oxygen
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures Lecture 14 mo theory Pdf

Solved The Formaldehyde Molecule Is Shown Below And We W Chegg Com

Molecular Orbital Theory

B He 2 2 S 2 2 S 2 2 Px 2 2 Py 2 C Hehe 2 S 2 2 S 2 2 Pz 2 2 Px 2 D Hehe 2 S 2 Course Hero
Http Butane Chem Uiuc Edu Pshapley Genchem2 A6 Book Pdf
2

Why There Is Difference In The Energy Levels Of O2 And N2 2px 2py And

Q1 Which Of The Followin Quotes Writings By Gagan Singla Yourquote

Which Of The Following Molecular Orbitals Has Two Nodal Planes

Molecular Orbital Electronic Configuration For X Anion Is Kk S2s S 2s

Number Of Nodal Planes In P 2px Are
Q Tbn 3aand9gcsn Bto2 Dbbzloy3qwhmmrutip17agfbrq6znnkj Tgoe6ekr3 Usqp Cau

2 Which Of The Following Overlaps Is Incorrect Assuming Z Axis Is Internuclear Axis 1
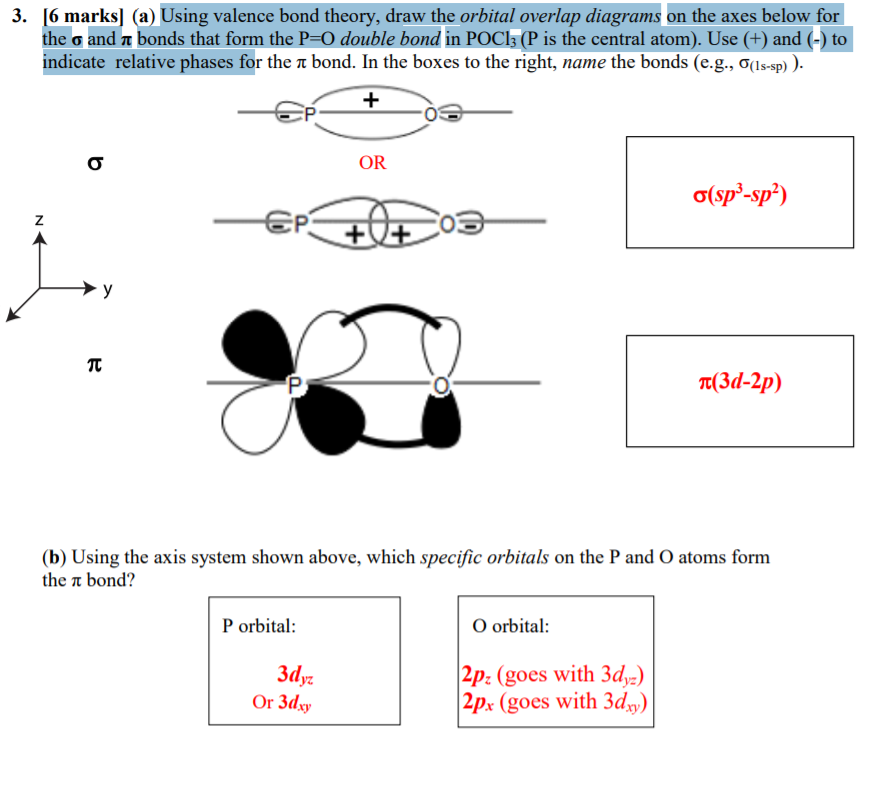
Solved Can Anyone Explain How They Got Sp2 And How They G Chegg Com
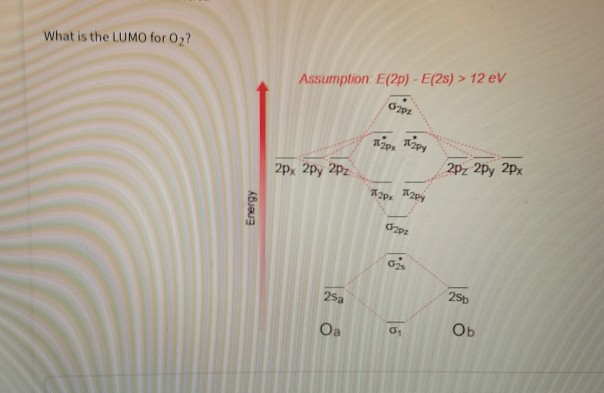
Solved The First Picture Is The Question And The Second P Chegg Com

1 A The Atomic Orbitals S And P Of Carbon And B The Molecular S Download Scientific Diagram
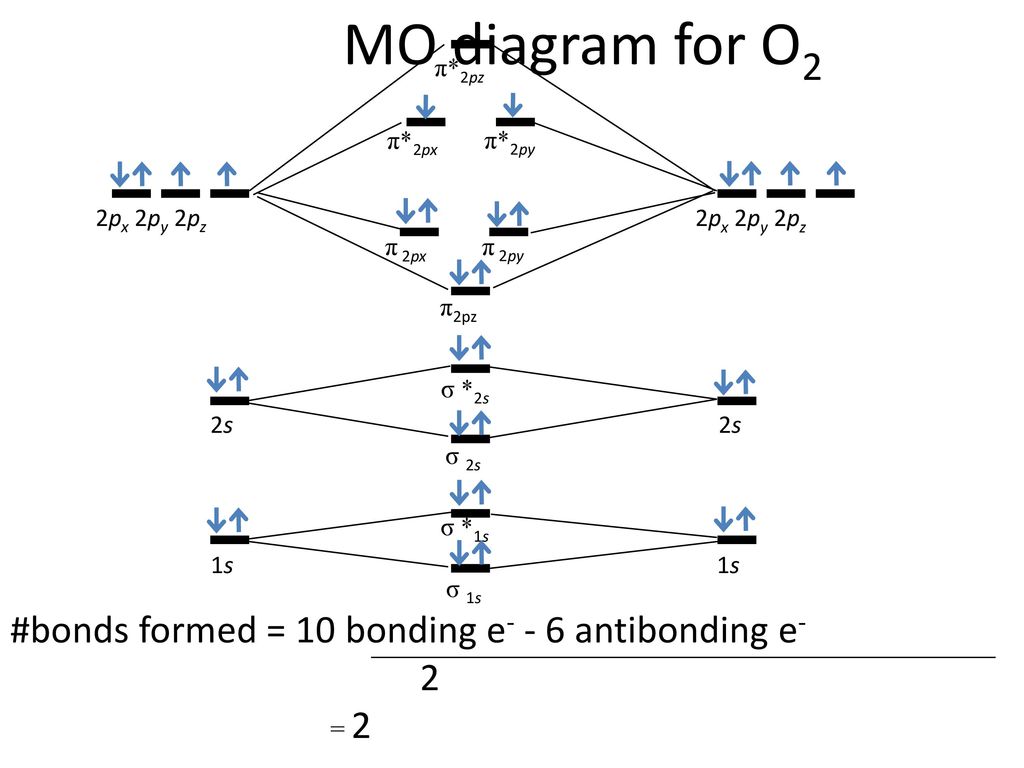
Advanced Organic And Mechanisms Ppt Download
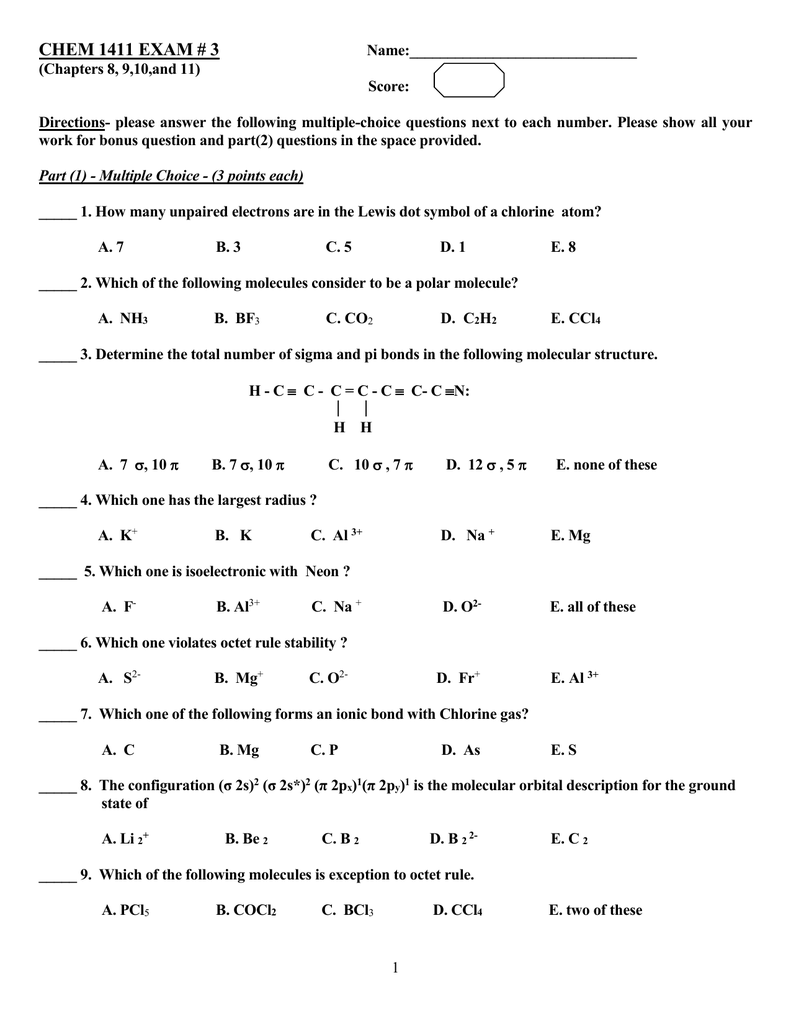
Exam 3practice Doc

Null D Lle On An Emple 7 If P Q R Are In A P Then 2px
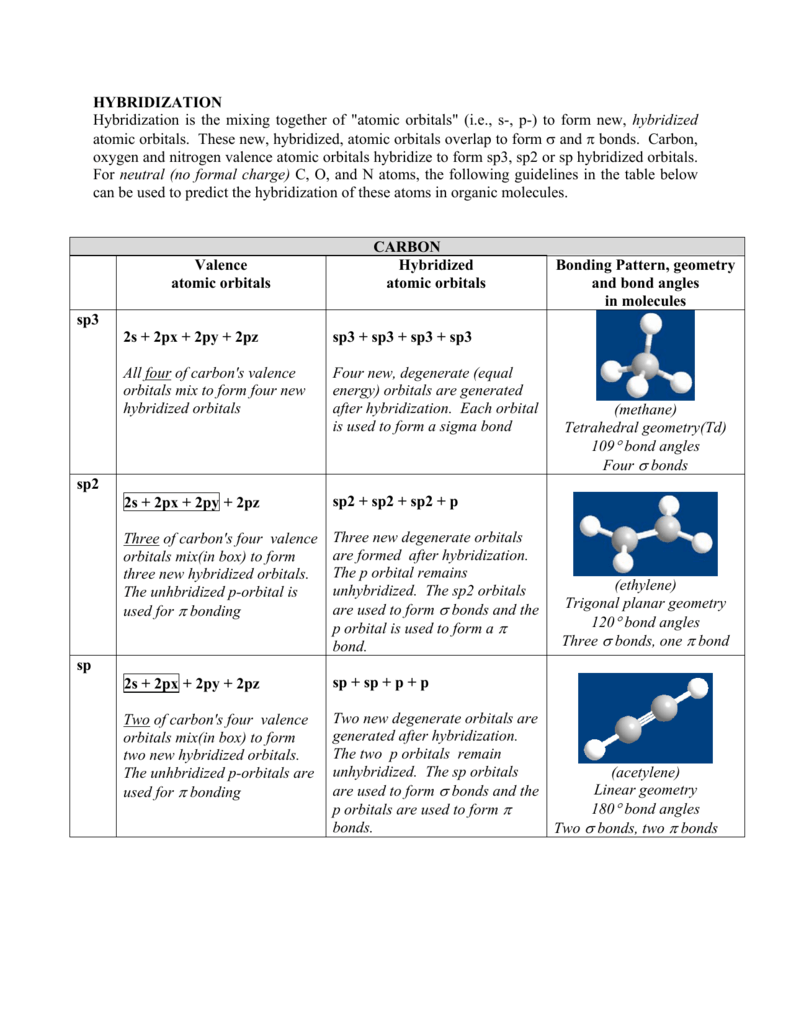
Hybridization Hybridization Is The Mixing Together Of Atomic
Cdn1 Byjus Com Wp Content Uploads 05 Ncert Exemplar Solutions For Class 11 Chemistry Ch 4 Chemical Pdf
Ciet Nic In Moocspdf Chemistry01 Kech Etext Pdf

According To Valence Bond Theory Oxygen Have No Free Electrons Still It Is Paramagnetic Explain The Brainly In

Solved 9 2 Ps Write The Molocular Orbital Cnargy Diagr Chegg Com
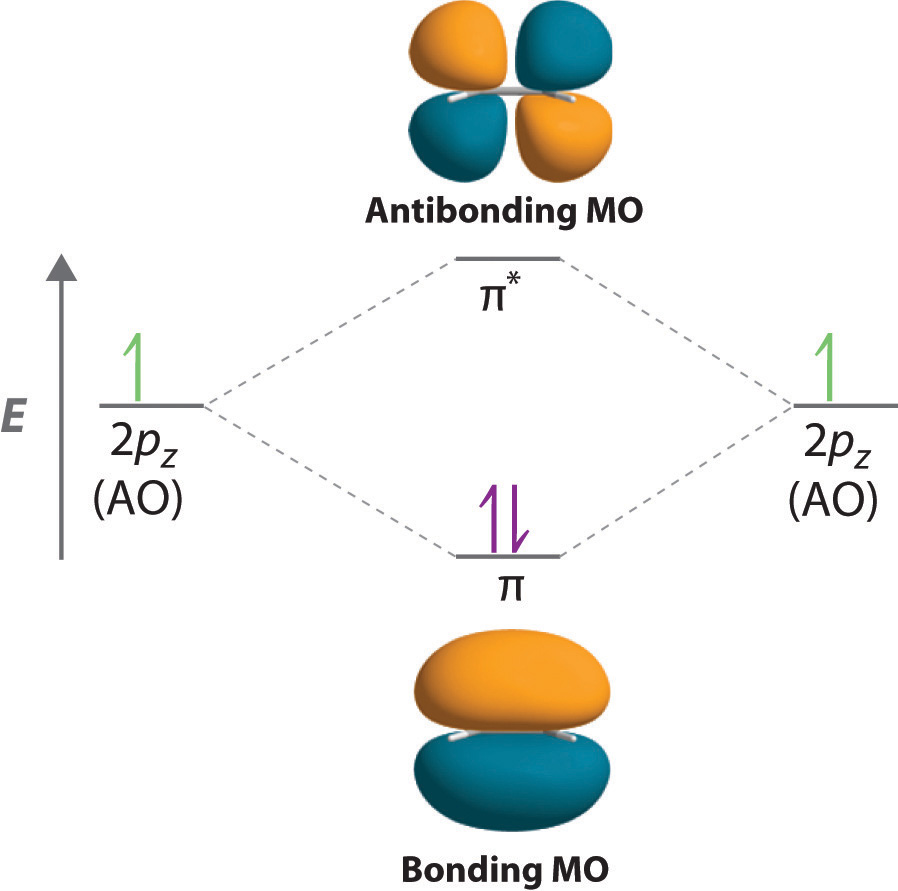
Molecular Orbital Energy Of Antibonding M O Is Higher Than The Energy Of Atomic Orbitals
Www Dalalinstitute Com Wp Content Uploads Books A Textbook Of Inorganic Chemistry Volume 1 Atoicv1 11 0 Metal Pi Complexes Pdf

L07 Chem 111a Lecture Notes Fall 15 Lecture 37 Antibonding Molecular Orbital Electronegativity Electron Configuration

L07 Chem 111a Lecture Notes Fall 15 Lecture 37 Antibonding Molecular Orbital Electronegativity Electron Configuration

What Are The Molecular Orbital Configurations For N 2 N 2 2 N 2 N 2 And N 2 2 Socratic
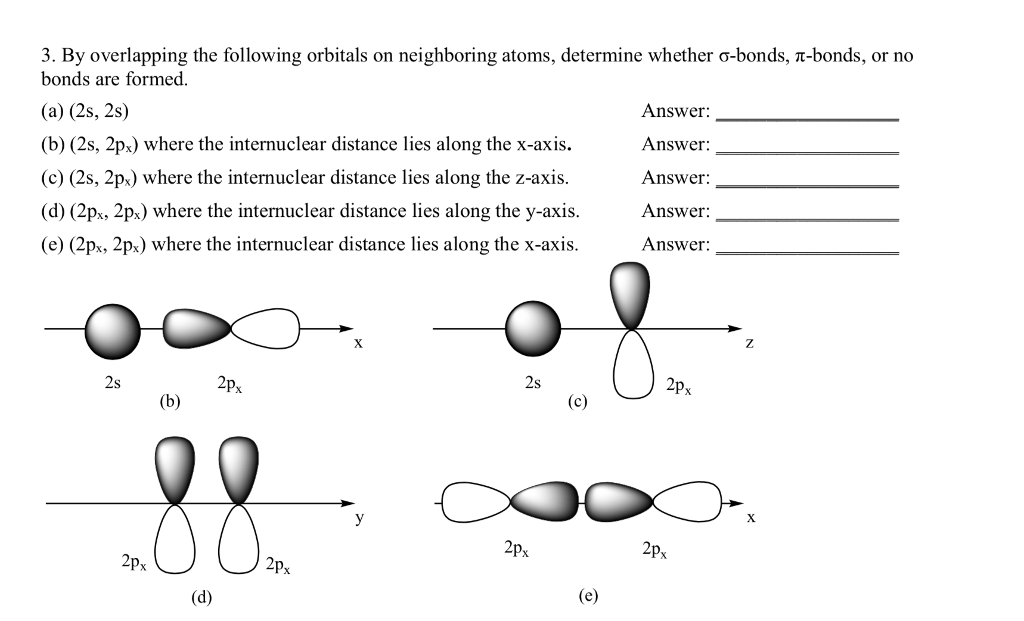
Solved 3 By Overlapping The Following Orbitals On Neighb Chegg Com
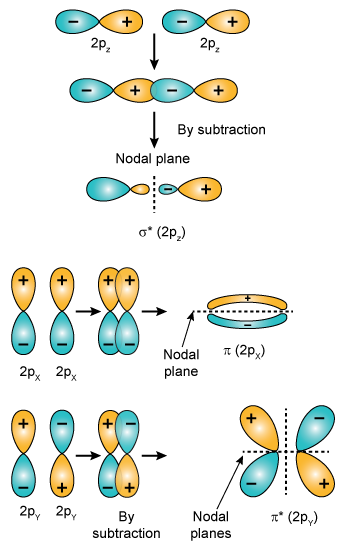
Which Of The Following Molecular Orbital Has Two Nodal Planes 1 2py 2 2s 3 2py 4 2pz Chemistry Topperlearning Com Yil5grcc
People Wou Edu Postonp Ch221 Pdf Summary Mo Theory W16 Pdf
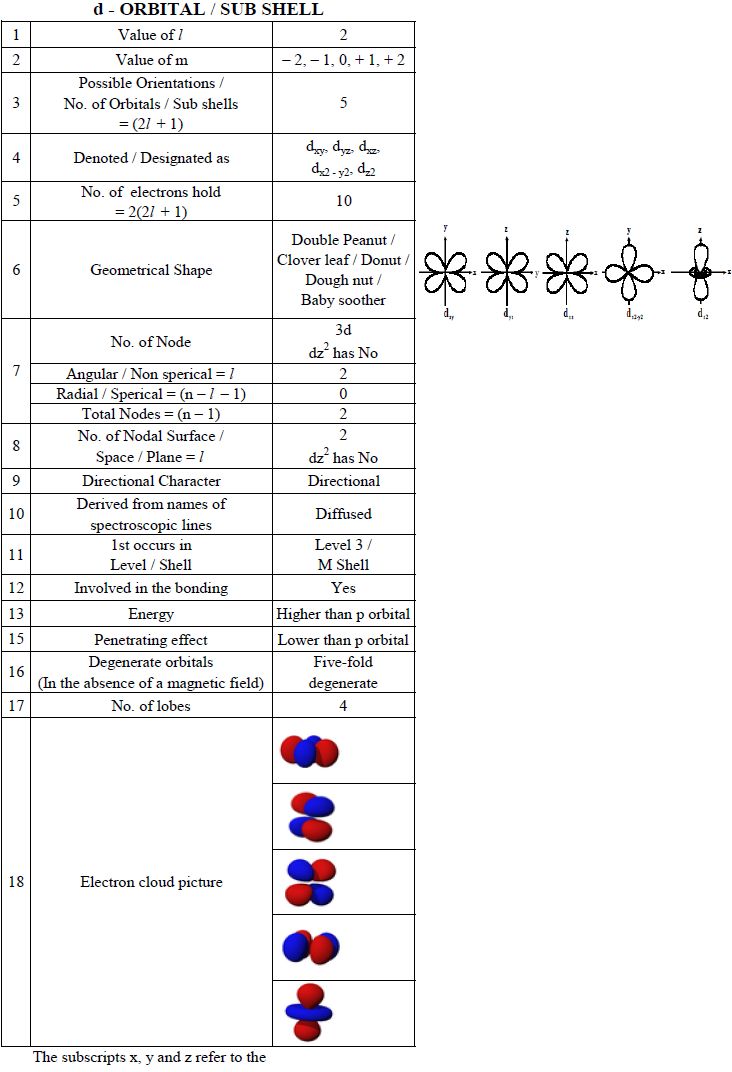
Chem Brains Atomic Structure Ii Five Marks

Chapter 9 Exercise Answers 19 Chem 121 Ubc Studocu

P 2px And P 2px Mo S Have One Nodal Plance Each
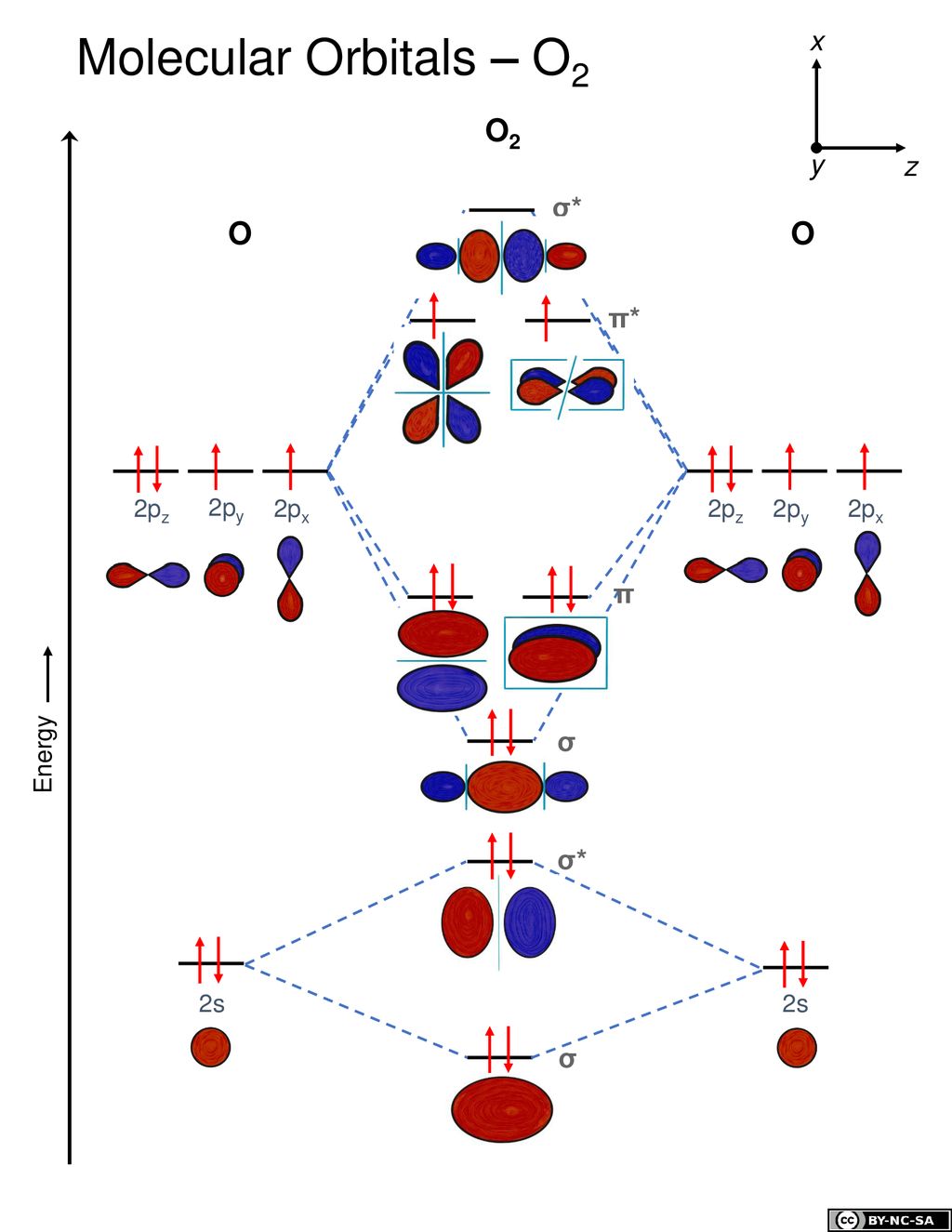
Molecular Orbital Theory Ppt Download
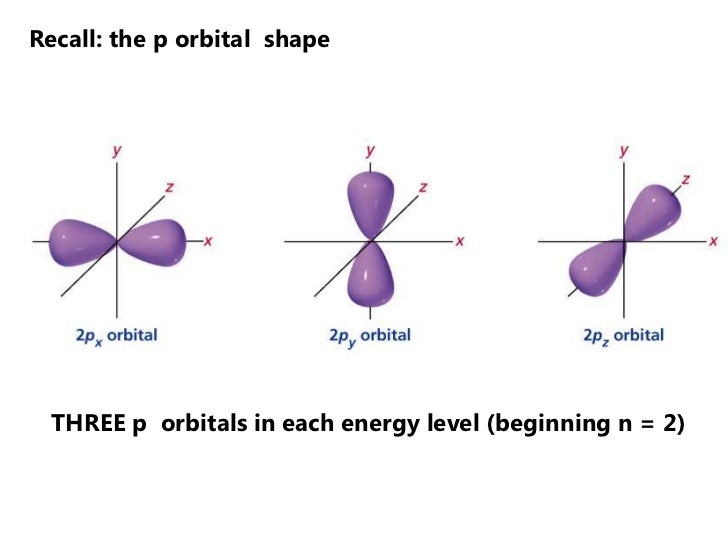
12 Orbital Hybrization Sigma And Pi Bonds

Molecular Orbital Theory Molecular Orbital Theory Describes Covalent Bonds In Terms Of Molecular Orbitals Which Result From Interaction Of The Ppt Download
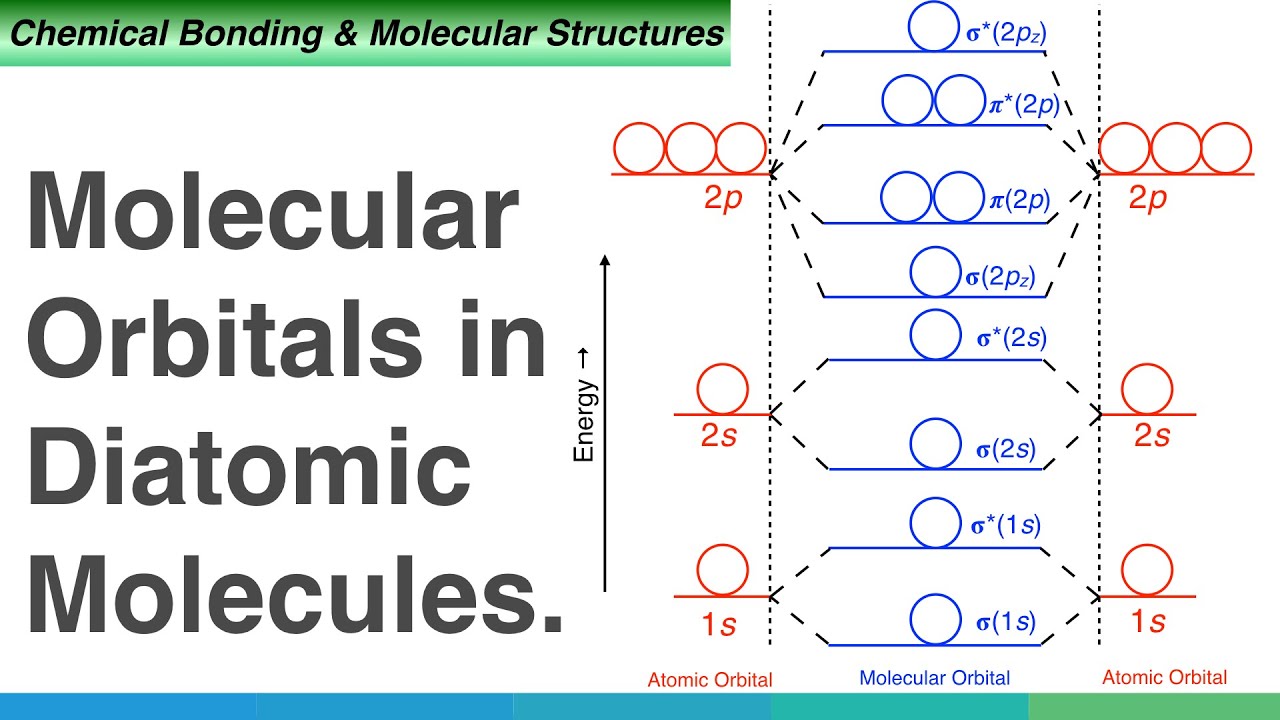
Molecular Orbitals In Diatomic Molecules Youtube

Draw Molecular Orbital Energy Diagram Of O2 Molecule And Mention Its Bond Order Explain Why O2 Brainly In

Draw The Molecular Orbital Formed On Sideways Overlap Of 2px With 2px



